< Electronics
Electronics | Foreword | Basic Electronics | Complex Electronics | Electricity | Machines | History of Electronics | Appendix | edit
Cells
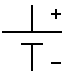
- Cell: Two materials with a voltage difference between them. This causes current to flow, which does work. Electrons travel from the cathode, do some work, and are absorbed by the anode.
- Anode: Destination of electrons.
- Cathode: Source of electrons.
- ions: An atom with an imbalance of electrons.
- cell operation: The cell runs and electrons are depleted at the cathode and accumulate at the anode. This creates a reverse voltage which stops the flow of electrons.
- irreversible: At some point the voltage difference reactions between the cathode and anode will decrease to a point that the cell in unusable. At this point, in an irreversible cell, the voltage difference is irreplaceably lost, and the cell is of no further use.
- reversible: Able to run the cell backwards.
- rechargeable: In a rechargeable cell, when the voltage difference between the cathode and anode decreases, the cell can be recharged, thereby increasing the voltage difference to a suitable level to allow continued use.
- humid air will discharge cells.
- cells are usually made of toxic or corrosive substances, for example lead and sulphuric acid. Such substances have been known to explode.
What is the relationship between voltage and electronegativity?
- Electronegativity is a concept in chemistry used to measure and predict the relative likelihood of a chemical reaction causing electrons to shift from one chemical to another resulting in ions and molecular bonds. A battery cell operates by allowing two chemicals to react and supply ions to the anode and cathode. When the supply of a reactant is consumed, the battery is dead. It no longer produces different electrical potential at the anode and cathode driven by the chemical reaction.
- Voltage is the electrical potential of a point due to surrounding measurable electric charge distributions and points as calculated by application Coulomb's Law. Voltage difference between two points connected by a conductor results in electron flow.
This article is issued from Wikibooks. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.